KOLON TISSUEGENE - Knee Osteoarthritis Study
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center, Phase 3 Study to Determine the Efficacy of TG-C in Subjects with Kellgren and Lawrence Grade 2 or 3 Osteoarthritis of the Knee
___________________________________________________________________
WEBSITE LINK for patient
STUDY PROTOCOL can be found here
STUDY PRODUCT: TG-C (Tissue Gene-C (3:1 mixture of non-transduced allogeneic human chondrocytes and irradiated transduced allogeneic human GP2-293 cells expressing TGF-β1)
TREATMENT GROUP:
ACTIVE GROUP (TG-C): 340 subjects will receive a single injection of TG-C at a dose of 3.0 × 107 cells.
PLACEBO: 170 subjects will receive a single injection of normal saline.
RANDOMINZATION: 2:1 (active drug vs. placebo) after stratification by KLG (2 vs. 3) and by gender
DOSAGE & ADMINISTRATION: Single intra-articular injection to the damaged joint area via ultrasound guidance
STUDY DURATION:
48 months (including start-up, enrollment/treatment, follow-up, and close-out)
Annual cancer surveillance questionnaires through 15 years post dosing for subjects who do not consent to participate in the separate 15-year Long-Term Safety Study.
PRIMARY OBJECTIVE: Evaluate the efficacy of TG-C with regard to knee function via WOMAC® total score and VAS pain score at 12 months
BLINDING: Everyone is blinded except for medication preparer and the injector
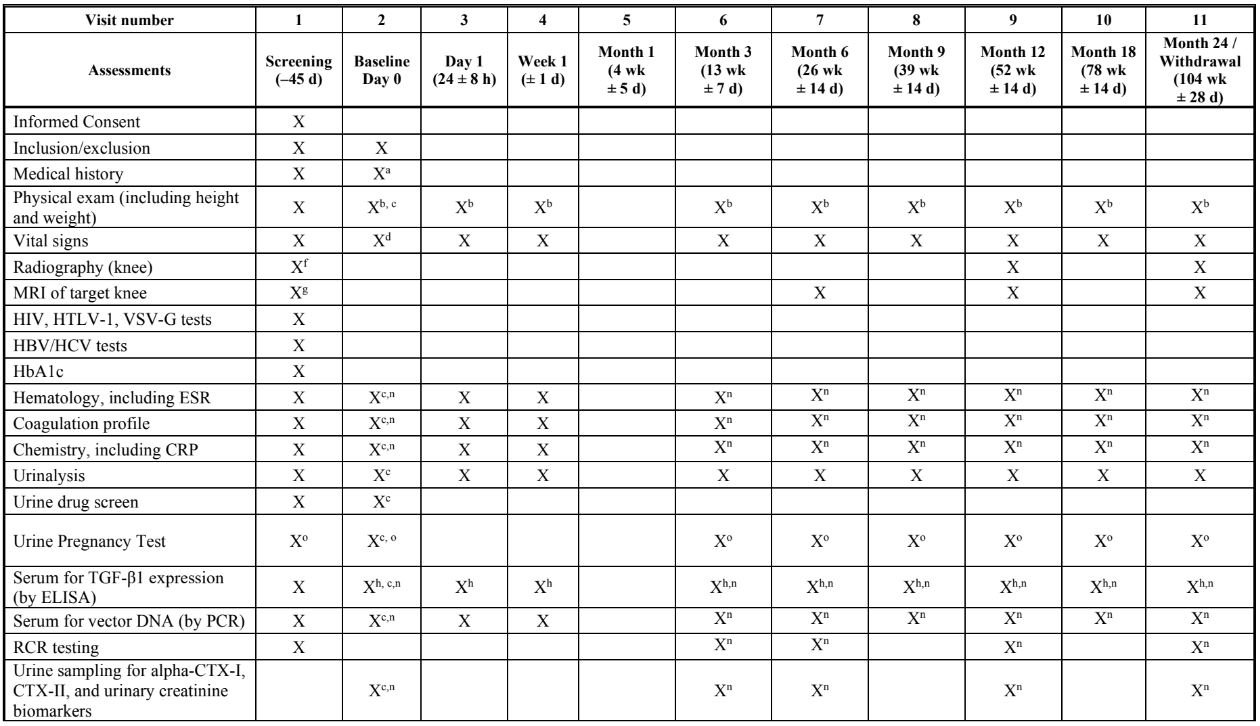
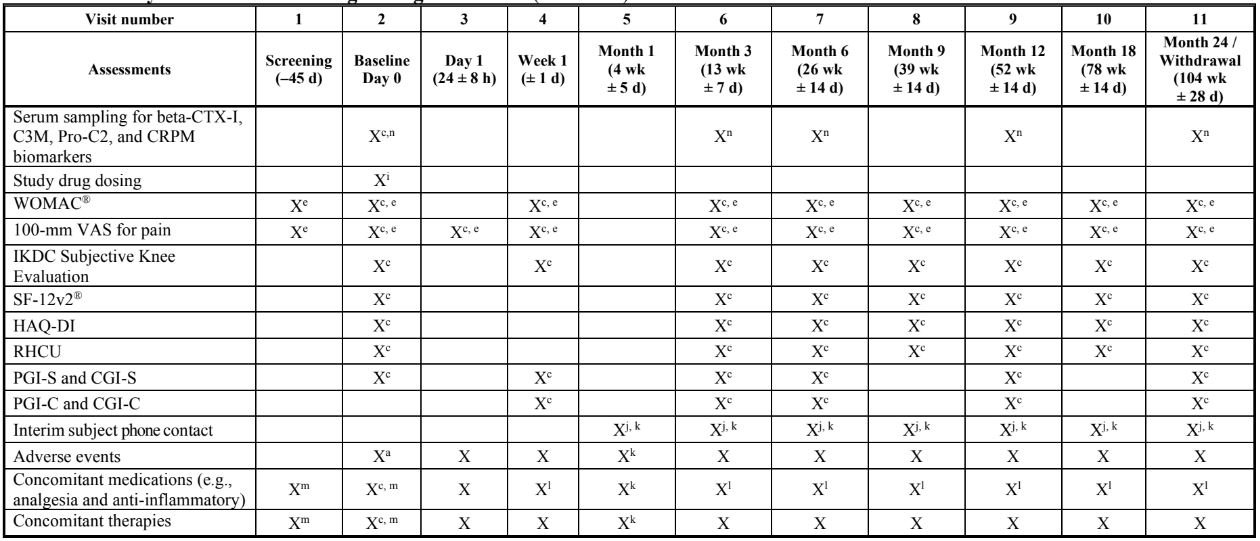
STUDY SCHEDULE:
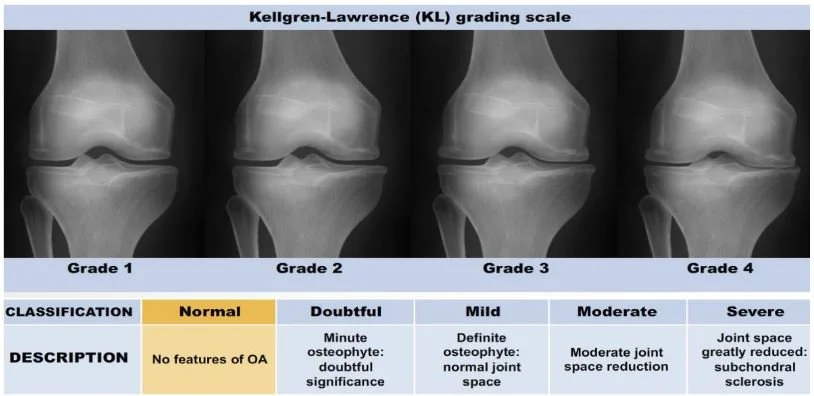
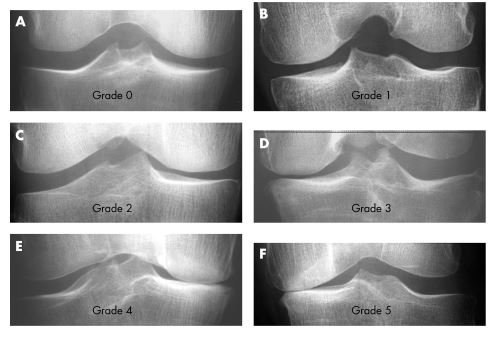
2. JSN Grades
3. RCR Testing
4. Section 7.2 (protocol on contraception requirement
5. Section 5.4 - Prohibited Medications / Drug Washout