A Clinical Utility Study of PrismRA Testing Therapeutic Response for Rheumatoid Arthritis (DRIVE)
STUDY PROTOCOL can be found here
STUDY PRODUCT:
TREATMENT GROUP:
DOSAGE & ADMINISTRATION:
Dosage and selection of b/tsDMARD are at the Investigator’s discretion but should be within the FDA approved label.
Dose adjustments for a particular b/tsDMARD will be permitted only in accordance with the FDA approved label. Changes should be documented in the patient’s medical records.
STUDY DURATION:
PRIMARY OBJECTIVE:
To stop disease progression, prevent irreversible joint destruction, and improve quality of life. Ideally, rheumatologists consult with their patients to decide whether disease remission (the absence of signs or symptoms of significant inflammatory disease) or low disease activity is the appropriate goal of treatment. After therapy is started, RA disease activity is measured regularly utilizing one of several validated tools (e.g., DAS, CDAI). Using the treat-to-target approach, therapeutic adjustments are made each visit to ensure continued progression to the agreed treatment target.
STUDY SCHEDULE:
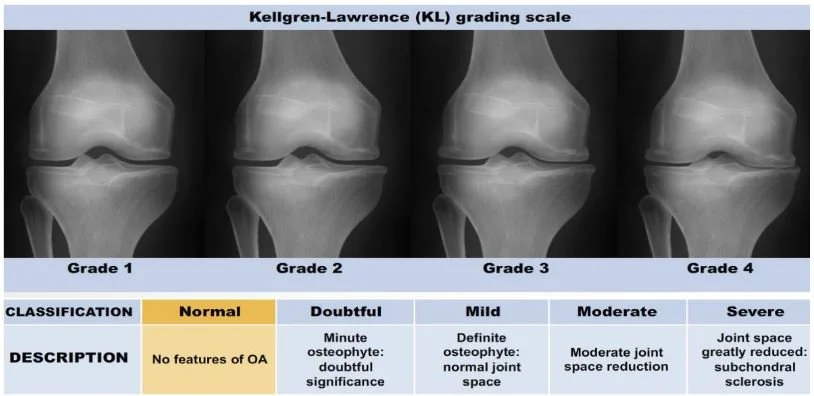
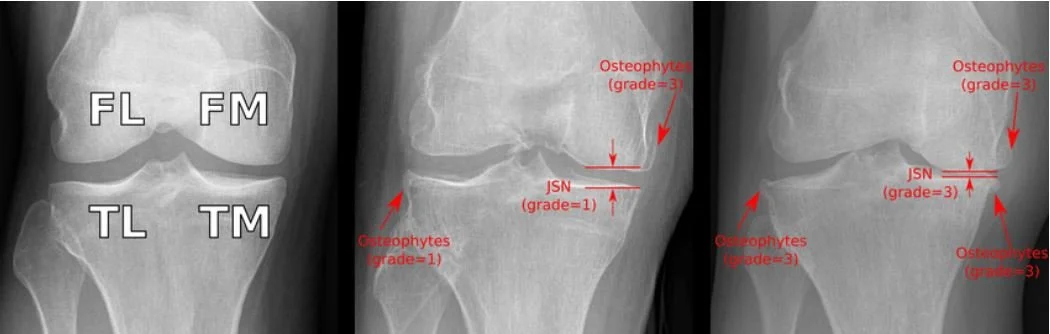
2. JSN Grades